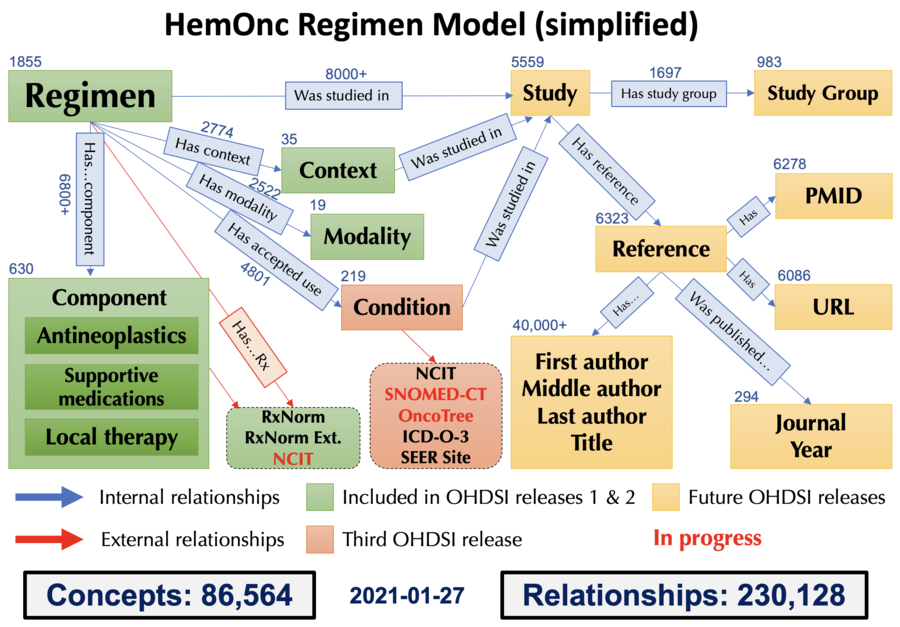
HemOnc vocabulary concepts
This page has short descriptions and examples of the concepts that have been parsed into the HemOnc ontology. Note that this is NOT the full HemOnc chemotherapy regimen model, as there are still concepts that are in the process of being defined and parsed. Also note that only some of these concepts are available thru OHDSI; these are noted below. For an overview and information about how to obtain a copy of the ontology please go to this page. You can also follow this link to the Relationships page.
Condition-level concepts
Condition
A condition is analogous to a diagnosis, and can be more or less granular. For example, Colon cancer and Rectal cancer are conditions, whereas Colorectal cancer is a condition that subsumes the other two. All conditions are hierarchically gathered into a top-level condition category.
Examples: acquired coagulopathy, acute myeloid leukemia, adrenocortical carcinoma
- Example of relationships: Colon cancer is a colorectal cancer is a gastrointestinal cancer is a malignant solid neoplasm is a condition
Context
| Available in OHDSI |
Context refers to the disease setting in which a treatment is administered. There are currently 33 contexts described in the HemOnc vocabulary, organized into a hierarchical structure with is a relationships.
Examples: adjuvant therapy, non-curative first-line induction therapy, upfront therapy
- Example of relationships: adjuvant therapy is a post-definitive therapy is an upfront therapy is a curative therapy is an all lines of therapy
Protocol-level concepts
Protocol
A protocol is the ordered combination of one or more Regimens. We are in the process of introducing this concept into the vocabulary - stay tuned!
Regimen-level concepts
Cycle Sigs
The timing of regimen cycles is captured as separate and distinct from the individual drug SIG of each of the components of the regimen. These so-called "cycle sigs" are a property of the regimen, although there are some cases where different components of a regimen have different cycle sigs (e.g., chemoradiotherapy often has different timing parameters for the chemotherapy and the radiotherapy parts). In the OMOP version of the HemOnc vocabulary, these instructions are captured as character strings; we are also in the process of digesting them into component parts.
Examples: 14-day cycle for up to 8 cycles, 21-day cycle for 4 cycles, 21-day cycle for up to 35 cycles (2 years)
Modality
| Available in OHDSI |
Modality refers to the intended effect of the agents being used in the regimen. Note that this can be distinct from the mechanism of action. The classic example is rituximab, which is a chemotherapeutic in the regimen R-CHOP and an immunosuppressive agent when used as monotherapy for immune thrombocytopenia. Other examples are steroids, which can be hormonotherapy, chemotherapy, or supportive medications depending on the intended use. We follow the SEER/NAACCR guidelines for modality, which means e.g., that a chemoradiotherapy regimen will have two modalities (chemotherapy and radiotherapy). In the future, we will develop a combined modality concept class.
Examples: anticoagulation, chemotherapy, immunotherapy
Regimen
| Available in OHDSI |
A regimen is one or more components given concurrently with an intended disease-modifying effect.
Examples: 7 plus 3d, 7 plus 3d and Midostaurin, R-CHOP
Regimen Stub
A regimen stub is something that would ordinarily be a regimen, but is missing details such as what are the individual components. The most common reason for this is that the regimen is described on HemOnc.org as a comparator to another regimen but there are no further details. This situation most commonly arises when an experimental comparator arm is not superior to the control arm, thus not meeting the criteria for inclusion on HemOnc.org.
Examples: FULV and Trimetrexate, Plitidespin and Dexamethasone
Regimen Class
A regimen class is a collection of regimens based on a common theme. Regimen classes enable the aggregation of different but similar regimens, and are often referred to within FDA labels.
Examples: platinum doublet, bevacizumab-containing regimen
Component-level concepts
Brand Name
| Available in OHDSI |
These are US and non-US brand names for drugs.
Examples: Abatoarin, Acetisal, Aclacin
Component
| Available in OHDSI |
Components are the granular constituents of regimens. The majority of components in the HemOnc ontology are drugs or biologics, along with a small number of radiation therapy components. In general we use the plain form name of a drug, not the conjugated salt or ester (e.g., sunitinib, not sunitinib malate; doxorubicin, not doxorubicin hydrochloride).
Examples: abciximab, abemaciclib, abexinostat
Component Class
| Available in OHDSI |
Most drug components in HemOnc are members of one or more component classes. These classes are organized primarily by mechanism of action. There are also several classes which are not mechanistic but may be of interest, e.g., WHO Essential Cancer Medicines.
Examples: 5 alpha-reductase inhibitor, AKT1 inhibitor, anthracycline
Procedure
| Available in OHDSI |
Surgical procedures generally performed with the intent of destruction or removal of cancerous tissue.
Examples: adrenalectomy, lymphadenectomy, interval debulking surgery
Rad Sig
These are dispensing instructions specific to radiation oncology prescriptions. We are in the process of breaking down these character strings into constituent parts.
Example: 110 cGy fractions x 70 fractions, given twice per day, 5 times per week (total dose: 7700 cGy)
Route
| Available in OHDSI |
These are the routes by which medications may be administered or taken.
Examples: intravenous, oral, subcutaneous
Sig
These are the dispensing instructions specific to drug prescriptions. In order to quality as a full SIG (as opposed to Sig Stub, see below), the instruction must contain the following: dose, dose unit, route, date. There are in addition several optional parameters, which will be shortly described in further detail. We are in the process of breaking down these character strings into constituent parts.
Example: 0.4 mg/m^2/day IV continuous infusion over 96 hours, started on day 1 (total dose per cycle: 1.6 mg/m^2)
Sig Stub
These are the dispensing instructions specific to drug prescriptions, that are lacking at least one of the four required categories to be designated a full SIG.
Example: (dose not specified) IT once per day on days 22 and 43
Study-level concepts
Clinical trial ID
When available (sometimes for trials published 2005-2015, nearly ubiquitous thereafter) this is the unique clinical trial ID for the study. Most often it is an NCT identifier, but can also be ISRCTN, Japa-CITI, EudraCT, etc.
Examples: ISRCTN96397434, NCT00058201, UMIN000000655
Endpoint
These are endpoints reported by the study, typically the primary endpoint when the study is "negative" and the least surrogate endpoint with P-value <= 0.10 when the study is "positive" for the primary endpoint. More information is [Response to treatment|available here].
Examples: CR rate after first induction, DFS36, OS, OS50%
- DFS36 = Disease Free Survival at 36 months (fixed interval endpoint)
- OS50% = median Overall Survival (fixed event endpoint)
Experimental design
These are the designs used by the experimental arm(s) in a study. More information is available here.
Examples: de-escalation, escalation, in-class switch, out-of-class switch
Study
This is the name of a prospective study conducted to evaluate one or more regimens on a population of human subjects. When possible, we try to use the abbreviated name provided by the authors and/or by ClinicalTrials.gov; these were somewhat uncommon prior to 2010 except for cooperative group studies. However, there are several examples where the same study name is used in different cancer types (e.g., BEACON) and we rename these to avoid name collisions. When no name is available, the default convention is to use the last name of the first author of the first publication describing the study, followed by et al., followed by year.
Examples: ABSOLUTE, ACOSOG Z1031, ECOG E3483, Pipas et al. 2005
Study Class
Right now, the only study class in HemOnc is the FDA registration study.
Example: FDA registration study
Study Group
A formal consortium of individuals or institutions assembled for the purposes of running large multi-site studies.
Examples: Eastern Cooperative Oncology Group, EINSTEIN Investigators, EORTC Children Leukemia Group
Publication-level concepts
Journal
The peer-reviewed journal in which a study is published, using the standard MEDLINE abbreviations.
Examples: Blood Adv, N Engl J Med, Ann Oncol
PubMedURL
Most of the references included in the HemOnc vocabulary are also indexed in MEDLINE. When this is the case, we also include a direct hyperlink to the PubMed abstract. Note: we plan to replace these URLs with PMID, soon.
Example: https://pubmed.ncbi.nlm.nih.gov/10202164
PubMedCentralURL
Some references included in HemOnc are deposited in PubMed Central and thus freely available to any user. Note: we plan to replace these URLs with PMCID, soon.
Example: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2409217/
Reference
This is the name for a published report on a study, using the following naming convention: Study::Sequence, where Sequence == 00 is the primary publication, Sequence == 01 is the first update, etc.
Examples: ABSOLUTE::00, Abrey et al. 2000::01
ReferenceTitle
This it the title of the published report on a study.
Example: A comparative study of two regimens of combination chemotherapy in acute leukemia
ReferenceURL
This is the direct hyperlink to the publication described by the Reference concept.
Example: https://ar.iiarjournals.org/content/31/6/2297.long
Author-level concepts
Author
Author names, in the format of Last-Name_First/Middle.
Examples: Abbruzzese_Alberto, Abbruzzese_James L, Abernethy_Amy
Generic concepts
Duration
This is a time concept that is applicable at the drug administration, regimen, and protocol level. For units of time one whole day or longer, the common unit of measurement is days. For units of time that are not whole days or are less than one day, the common unit of measurement is hours.
Examples: 0.25 hours, 1 day, 90 days
Null
This is the generic absence of something.
Numeric
This is a unitless value that can represent concepts such as number of cycles
Year
Examples: 1946, 2020